Support » Pololu 3pi Robot User’s Guide » 5. How Your 3pi Works »
5.a. Batteries
Introduction to Batteries
 |
Two rechargeable AAA Ni-MH batteries. |
|---|
The power system on the 3pi begins with the batteries, so it is important to understand how your batteries work. A battery contains a carefully controlled chemical reaction that pulls electrons in from the positive (+) terminal and pushes them out of the negative (-) terminal. The most common type is the alkaline battery, which is based on a reaction between zinc and manganese through a potassium hydroxide solution. Once alkaline batteries are completely discharged, they cannot be reused. For the 3pi, we recommend rechargeable nickel-metal-hydride (NiMH) batteries, which can be recharged over and over. NiMH batteries are based on a different chemical reaction from alkaline batteries, but you don’t need to know anything about the chemical details to use a battery: everything you need to know about it is measured with a few simple numbers. The first is the strength with which the electrons are pushed, which we measure in volts (V), the units of electric potential. An NiMH battery has a voltage of about 1.2 V. To understand how much power you can get out of a battery, you also need to know how many electrons the battery can push per second – this is the electric current, measured in amps (A). A current of 1 A corresponds to about 6×1018 electrons flowing out one side and in to the other each second, which is such a huge number that it’s easier to talk about it just in terms of amps. 1 A is also a typical current that a medium-sized motor might use, and it’s a current that will put a significant strain on small (AAA) batteries.
For any battery, if you attempt to draw more and more current, the voltage produced by the battery will drop, eventually dropping all the way to zero at the short circuit current: the current that flows if you connect one side directly to the other with a thick wire. (Don’t try this! The wire might overheat and melt, and the battery could explode.) The following graph shows a good model of how the voltage on a typical battery drops as the current goes up:
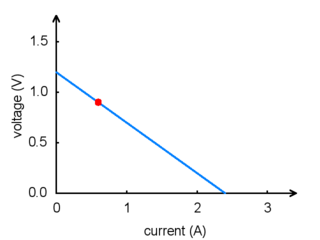 |
Battery voltage vs. current. |
|---|
The power put out by a battery is measured by multiplying the volts by the amps, giving a measurement in watts (W). For example, at the point marked in the graph, we have a voltage of 0.9 V and a current of 0.6 A, this means that the power output is 0.54 W. If you want more power, you need to add more batteries, and there are two ways to do it: parallel and series configurations. When batteries are connected in parallel, with all of their positive terminals tied together and all of their negative terminals tied together, the voltage stays the same, but the maximum current output is multiplied by the number of batteries. When they are connected in series, with the positive terminal of one connected to the negative terminal of the next, the maximum current stays the same while the voltage multiplies. Either way, the maximum power output will be multiplied by the number of batteries. Think about two people using two buckets to lift water from a lake to higher ground. If they stand next to each other (working in parallel), they will be able to lift the water to the same height as before, while delivering twice the amount of water. If one of them stands uphill from the other, they can work together (in series) to lift the water twice as high, but at the same rate as a single person.
In practice, we only connect batteries in series. This is because different batteries will always have slightly different voltages, and if they are connected in parallel, the stronger battery will deliver current to the weaker battery, wasting power even when there is nothing else in the circuit. If we want more current, we can use bigger batteries: AAA, AA, C, and D batteries of the same type all have the same voltage, but they can put out very different amounts of current.
The total amount of energy in any battery is limited by the chemical reaction: once the chemicals are exhausted, the battery will stop producing power. This happens gradually: the voltage and current produced by a battery will steadily drop until the energy runs out, as shown in the graph below:
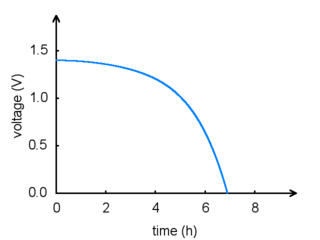 |
Battery voltage vs. time. |
|---|
A rough measure of the amount of energy stored in a battery is given by its milliamp-hour (mAH) rating, which specifies how long the battery will last at a given discharge rate. The mAH rating is the discharge rate multiplied by how long the battery lasts: if you draw current at a rate of 200 mA (0.2 A), and the battery lasts for 3 hours, you would call it a 600 mAH battery. If you discharge the same battery at 600 mA, you would get about an hour of operation (however, battery capacity tends to decline with faster discharge rates, so you might only get 50 minutes).
Note: If you have purchased rechargeable batteries for the 3pi, you should fully charge them before you first use them. You should never attempt to program your 3pi if its batteries are drained or uncharged. Losing power during programming could permanently disable your 3pi.






